Radiation Therapy
Overview
Radiotherapy is a procedure to treat disease using radiation. Radiation provokes DNA damages in cancerous cells and leads to cell death. While normal cells may also be affected by radiation, normal cells express a strong ability to repair the radiation-induced DNA damages. Radiotherapy can be used alone, or as an adjunct to chemotherapy, hormonal therapy, immunotherapy or surgery. Apart from malignant tumours, radiotherapy can also be used to treat benign tumours such as pituitary adenoma and keloid.
Linear accelerators that produce 4-25 MV high energy photon beams and electron beams are the most common apparatus for external beam radiation therapy. High energy photon beams can reach deep seated tumors such as nasopharyngeal cancer and prostate cancer. A 6 MV photon beam is suitable for most treatment applications because of its optimal penetrating power and its relatively low room shielding requirement. Electron beams are mainly applied to shallow lesions such as skin cancer.
The output of a linear accelerator is extremely stable. The radiation will stop emitting and not remain in the patient body after the linear accelerator is switched off. TrueBeam®, Radixact®, CyberKnife® etc. are types of linear accelerators available for external beam radiation therapy in Hong Kong. The oncologist will select the appropriate type of linear accelerator for treatment according to each patient’s condition.
Technique
Tumors in thoracic and abdominal regions, such as breast cancer, lung cancer and liver cancer, move as patient breathes. Additional margins are added to ensure the tumor/target coverage. However, the surrounding healthy tissues are inevitably irradiated at the same time. Respiratory motion management is therefore used to minimize the effect of breathing motion. It is particularly important in stereotactic body radiotherapy (SBRT) where extremely high dose is delivered.
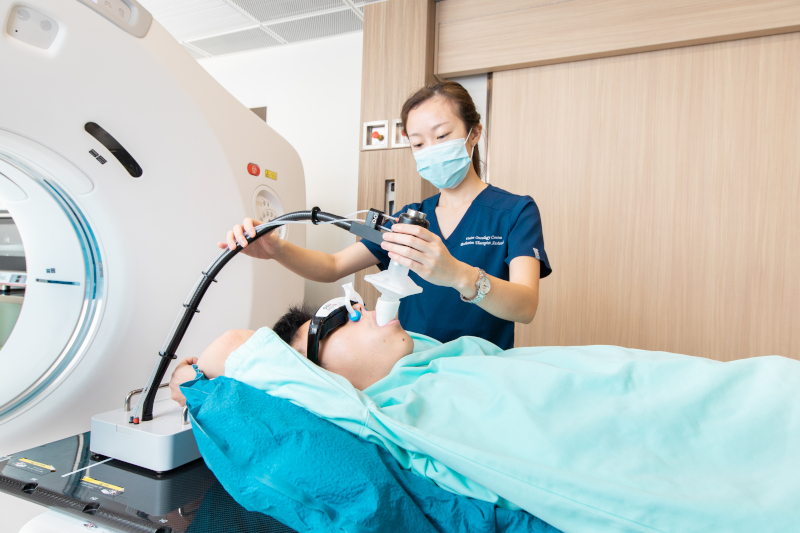
Our centre provides the following respiratory motion management techniques:
-
Deep-inspiration breath-hold (DIBH)
What is DIBH radiotherapy?DIBH radiotherapy is a technique of tumor motion management during radiotherapy simulation or treatment. Patient is instructed to voluntarily hold the breath after deep inspiration using a respiratory gating device.What is the benefit of using the DIBH technique for left breast radiotherapy?When patient hold the breath, lungs will inflate and displace the heart away from the left breast and chest wall. This can minimize the volume of the heart being irradiated and reduce the risk of potential damages to the heart.How to perform DIBH?A respiratory gating device will be used to help patient perform voluntary DIBH. It detects the breathing cycles and lung volume. A training session will be provided by radiation therapist to familiarize patient with the device. Patient will be given a disposable mouthpiece, an anti-bacterial filter, a nose clip, and video glasses. Patient can only breathe through the mouthpiece when using the respiratory gating device. The spirometer inside the device interprets the breathing cycles. It helps the radiation therapist decide the maximum optimal breath-holding level where patient can hold the breath for 20 seconds after deep inspiration. Patient can visualize his/her breathing pattern and breath-hold level using the video glasses. And radiation therapists will guide the patient to achieve maximum optimal breath-hold throughout the DIBH procedures.What happens during CT simulation with DIBH?Two sets of CT images will be acquired during CT simulation – with and without breath-hold. Patient will have an initial CT scan with free breathing. While in the second scan, he/she will be asked to perform breath-hold using the respiratory gating device. Most of the breath-hold CT scans can be completed within a single breath-hold in about 20 seconds.What happens during treatment delivery with DIBH?Radiation therapists will first position the patient according to the skin marks. Pre-treatment images will be acquired to verify treatment position before treatment. DIBH is required during imaging and treatment delivery. The procedures of DIBH in treatment delivery is very similar to those in CT simulation. The major difference is that patient is required to perform several breath-holds during treatment delivery because treatment delivery beam-on time is usually longer than CT scan time. Patient is allowed to take a short break between breath-holds. Radiation therapists in the control room will carefully monitor the breathing pattern and the treatment through the CCTV. The radiation beam will be switched off if the breath-hold is not satisfactory. Throughout the whole treatment, radiation therapists will communicate with patient via intercom and treatment delivery can be interrupted if the patient needs any assistance.
-
Active-breathing control (ABC)
Active-breathing control (ABC) is similar to DIBH, where a respiratory gating device connecting to a spirometer is used to help the patient perform breath-hold. ABC and DIBH differ in the threshold level. In ABC, we encourage patients to do moderate to deep breath-hold during inhale or exhale in a reproducible manner. We aim to achieve substantial and reproducible internal organ displacement while maintaining patient comfort during treatment. ABC is commonly used in lung cancer, liver cancer, or other thoracic/abdominal treatment.
-
Four-dimensional computed tomography (4DCT)
Image quality of conventional thoracic and abdominal CT scan is significantly reduced by the motion artifact introduced by the breathing motion. Not only will it be hard for the oncologist to outline the target accurately, but it also will affect the dose calculation accuracy of the radiation treatment plan. In 4DCT (or respiration-correlated CT) simulation, an infrared system will be utilized for detecting breathing motion. The system consists of an infrared camera and an infrared reflective marker that is placed on patient’s anterior abdominal surface. The infrared camera detects the respiratory motion signals reflected from the marker and correlates those signals to the CT images. Tumor range of motion can be visualized in the reconstructed CT images. This information is valuable for oncologist in defining optimal treatment margins for treatment planning. During treatment delivery, patient can breathe freely and maintain steady normal breathing which is easy for most of the patients. Therefore, 4DCT is suitable for most of the patients requiring thoracic or abdominal treatment.
-
Real-time respiratory gating
In real-time respiratory gating, beam delivery is controlled by breathing motion recorded by the infrared camera. During treatment planning, 4DCT is acquired to visualize tumor motion and helps oncologist determine which phase of the breathing cycle (the “gate”) is used for treatment. In other words, radiation beam is on only during the pre-defined phase of the patient’s breathing cycle. The advantage of respiratory gating is that the patient is able to breathe comfortably without external abdominal compression or breath-hold. Nevertheless, only those patients who can maintain regular breathing are suitable for respiratory gating radiotherapy.
-
Forced-shallow breathing by abdominal compression
Abdominal compression technique restricts normal breathing by using stereotactic body frame with an attached plate to press against the abdomen. A comfortable and reproducible position is crucial to the efficacy of respiratory management. During simulation, radiation therapists will apply different levels of pressure to patient’s abdomen in order to determine optimal and effective abdominal compression.
Respiratory motion management is effective in minimizing the uncertainties introduced by respiratory motion in thoracic and abdominal radiotherapy. Different techniques may be selected based on the treatment position, size of tumor, patient’s condition and preference, etc. Please discuss with your oncologist for the most suitable option. You may also ask our radiation therapists for details.
Stereotactic Radiosurgery (SRS) is used to treat intracranial benign or malignant tumours and abnormalities. In 1951, a Swedish neurosurgeon called Lars Leksell developed SRS as an alternative to open brain surgery, in which the mortality rate could be as high as 40%. Stereotactic radiosurgery is a non-surgical radiation therapy that delivers high radiation dose to the small brain tumour very precisely. While the number of fractions in a conventional radiation treatment course ranges from several sessions to 40 sessions, stereotactic radiosurgery is a one-fraction radiation treatment. SRS is called “surgery” because it can have the same result as precise and accurate as a single open surgery. In some instances, the radiation treatment is fractionated into several days. In that instance, it is called “Fractionated Stereotactic Radiotherapy” (FSRT).
SRS can treat diverse types of conditions such as meningioma, arteriovenous malformation (AVM), malignant brain tumor, brain metastasis, acoustic neuroma, trigeminal neuralgia, pituitary adenoma, etc. Lung cancer is the leading cause of cancer deaths in Hong Kong (data from Hong Kong Cancer Registry, 2017). Due to the recent advance development in chemotherapy and targeted therapy drug, patient’s survival rate and quality of life are greatly improved. SRS then becomes an important option for treating brain metastases in patients at late stages of lung cancer. In comparison to traditional whole brain radiotherapy (WBRT), the cognitive function can be better preserved by avoiding radiation beams to hippocampus in SRS.
The “small target, single session, precise and high dose” characteristics of SRS make it favorable to traditional external beam radiotherapy as it can minimize the radiation dose to the surrounding normal brain tissues, thus reduce the side effect. Every surgical planning requires the corporation among a professional team comprising clinical oncologists, neurosurgeons, medical physicists, radiation therapists and specialized nursing staff. During the SRS, the tumour is precisely located and treated using non-coplanar high energy focused radiation beams delivered by a SRS system. SRS is like a virtual surgical scalpel, that is, patients do not have any pain feeling or any risks from general anesthesia and open brain surgery.
From the mid-90s to 2010, circular collimators were commonly used to collimate a narrow beam from a linear accelerator in SRS treatments. As the technology of image-guided radiotherapy was not mature at that time, neurosurgeons would fit a stereotactic frame over the head of the patient with pins under local anesthetic, and then locate the tumor using 3-dimensional stereotactic technique during treatment. The stereotactic frame may be attached to the patient’s head for 6-7 hours between the planning phase and the actual treatment delivery of a single treatment target. Patients with multiple brain metastases of more than 12 targets would have to experience a process with headframe attached for over 20 hours. Such long duration of frame attachment may not be tolerable for some patients. Nowadays, giving thanks to rapid developments on high resolution (2.5 mm) multileaf collimator (MLC) for intensity modulated radiotherapy (IMRT), 6D treatment couch and image-guided radiotherapy technique, the efficiency and treatment accuracy are greatly enhanced.
Recently, most hospitals have employed frameless SRS. Patients feel more comfortable as they do not have to suffer from pain and wound because of attaching headframe with pins under local anesthetic. Some hospitals also employ real-time image guided technology (e.g. Brainlab ExacTrac® from Germany), which provides real time treatment position verification to increase the positional accuracy and treatment efficiency. Sub-millimeter accuracy can be achieved by tracking patient position using two in-room kV X-ray units and optical infrared tracking system (or the latest thermal surface tracking technology) with the 6D adjustable treatment couch. The treatment time is greatly shortened to 20–30 (SRS) minutes for treating a single target. In the instance of deviations from the prescribed target position during treatment, the system will alert radiation therapist for target re-alignment by adjusting the couch before continuing the treatment.
Besides linear accelerator, Gamma Knife® (Elekta AB, Stockholm) and CyberKnife® (Accuray, Sunnyvale, USA) are generally recognized devices for SRS or FSRT systems in radiation oncology.
To summarize, SRS and FSRT are useful in the management of benign and malignant intracranial lesions with its “small target, single session, precise and high dose” characteristics. Real time image guidance is complementary technology in SRS / FSRT treatments to further improve the accuracy.
HyperArcTM is an advanced end-to-end solution to deliver sophisticated, non-coplanar intracranial stereotactic radiosurgery (SRS) or stereotactic radiotherapy (SRT). By automating and simplifying the planning and treatment workflow, HyperArcTM revolutes stereotactic treatments to be safer, more accurate and efficient.
System Highlights:
-
Non-invasive and dedicated immobilization device
Frameless and high-precision immobilization system with sub-millimeter accuracy. Meanwhile, the open view thermoplastic device reduces patient anxiety and discomfort.
-
Multiple brain metastases simultaneous treatment
HyperArcTM treatment is designed to irradiate multiple lesions with a single isocenter without patient repositioning. Shortened treatment time reduces the chance of patient movement during treatment.
-
Automated intra-fractional imaging
During a complex non-coplanar SRS treatment, it is often challenging to mark a safe location for image verification. HyperArcTM addresses this by pre-determining a set of imaging waypoints in the delivery process.
-
One-click treatment delivery with pre-determined delivery sequence
Delivery sequences are optimized by the treatment planning system and derived from a superset of trajectories. HyperArcTM treatment can be delivered seamlessly with a single click of the console.
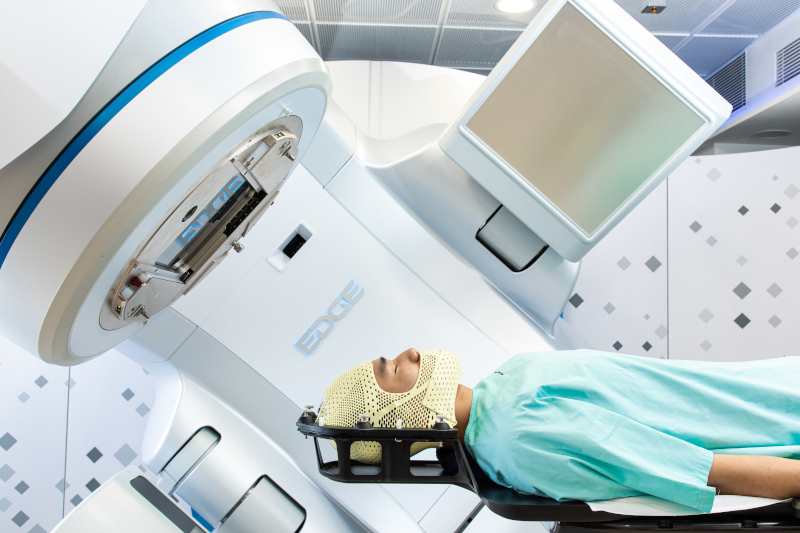
Intensity Modulated Radiotherapy (IMRT) is the most common radiotherapy technique in private hospitals in Hong Kong. IMRT is a special radiotherapy technique that controls the intensity of each radiation beamlet by treatment planning computer and multileaf collimator (MLC). IMRT optimizes the dose distributions to irregularly shaped tumours or tumours which are close to critical organs, so that the doses to surrounding critical organs could be minimized, thus treatment side effects are greatly reduced. IMRT offers dosimetric advantages over traditional radiotherapy in reducing acute and chronic side effects. For example, in the treatment of head and neck region, the doses to critical structures such as brain stem, spinal cord, esophagus, oral mucosa, tongue, parotid glands, optic nerves are highly reduced using IMRT. Moreover, it is essential for IMRT to equip with imaging modalities such as cone beam computed tomography (CBCT) to ensure treatment position accuracy. A team of well-trained healthcare professionals is also required to carry out IMRT treatments.
Volumetric modulated arc therapy (VMAT) can be considered as an extension of IMRT treatment technique that delivers radiation dose with varying dose rate as the gantry rotates around the patient. Varian Medical Systems introduced RapidArcTM for VMAT. The speed of gantry rotation, beam shaping aperture and dose delivery rate are simultaneously varied during treatment. Compared to IMRT, instead of gantry stopping at multiple beam angles, the treatment can be completed in 1-2 gantry rotations and the treatment time can be greatly reduced to a few minutes.
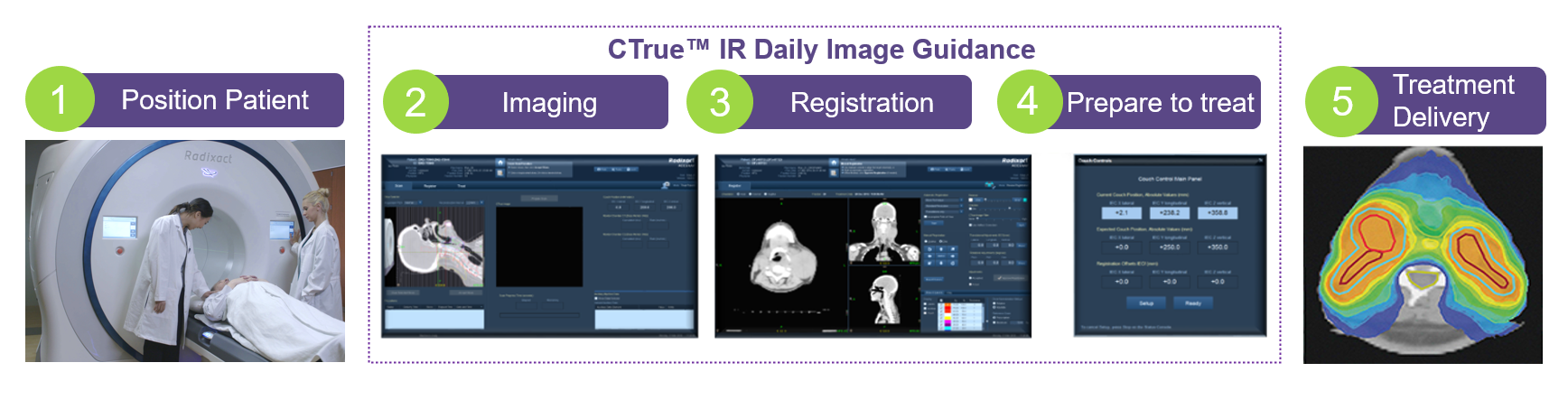
Image-guided radiation therapy (IGRT) integrates medical imaging into radiotherapy to improve localization and monitoring of tumor position, size and shape before and during the treatment, thus guides the delivery of radiation accurately.
At the beginning of a treatment session, radiation therapist will position the patient on the treatment couch according to the treatment plan with the aid of in-room lasers. Then, images are taken with an imaging system before or during the treatment. By comparing these images to the reference images taken during simulation, the patient's position and the radiation beams can be adjusted to irradiate to the tumor more precisely. Multiple images may be acquired for verification. The image guidance process is expected to take several minutes. Although it increases the total time in each treatment session, treatment accuracy can be improved with positional adjustment according to patient anatomy during treatment. In conclusion, IGRT is essential in radiotherapy treatments with high precision requirement, such as intensity modulated radiotherapy (IMRT), stereotactic radiosurgery (SRS), stereotactic body radiotherapy (SBRT).

Stereotactic Body Radiation Therapy (SBRT) or, Stereotactic Ablative Radiotherapy (SABR) is a radiotherapy technique for treating cancer at the lungs, head, prostate and liver. It is a non-invasive treatment procedure with a high dose prescription (can be up to 30 Gy/fraction) in relatively few fractions (1-8 fractions). The normal prescription dose in a conventional radiotherapy is around 2 Gy/fraction, the prescription dose per fraction in SBRT can be 15 times higher than that of conventional radiotherapy. Therefore, it is necessary to use special radiotherapy equipment to ensure the precision of high dose delivery to a small tumour region.